Services
The Herston Biofabrication Institute provides biomedical design and engineering services to clinicians using 3D modelling and 3D printing in adherence with the medical device regulatory framework. HBI specialises in personalised medical devices, including patient-matched anatomical models for surgical planning, as well as custom-made medical devices.
We also collaborate with clinicians and academics to research, design, develop and manufacture advanced education and training models, and other tools. Please use the forms below to place a referral or a support request.
Request forms
Metro North Health is the sponsor and manufacturer of medical devices at HBI in accordance with the Therapeutic Goods (Medical Devices) Regulations 2002. Our medical device production takes place under a quality management system.
You can also contact our Biomedical Engineers at: hbidevices@health.qld.gov.au.
Frequently requested
Custom-made medical devices: orthoses, bone cement mould, tissue reconstruction aids.
Clinically-applied 3D printing
Metro North HHS, with the University of Queensland and research, industry and government partners, are partnering through the Herston Biofabrication Institute by co-locating engineering and medicine expertise to develop new medical devices and bioengineered constructs with a direct path to translation in clinical practice. It also creates new educational pathways for future engineers and clinicians, as well as upskilling opportunities for the current workforce around surgical engineering.
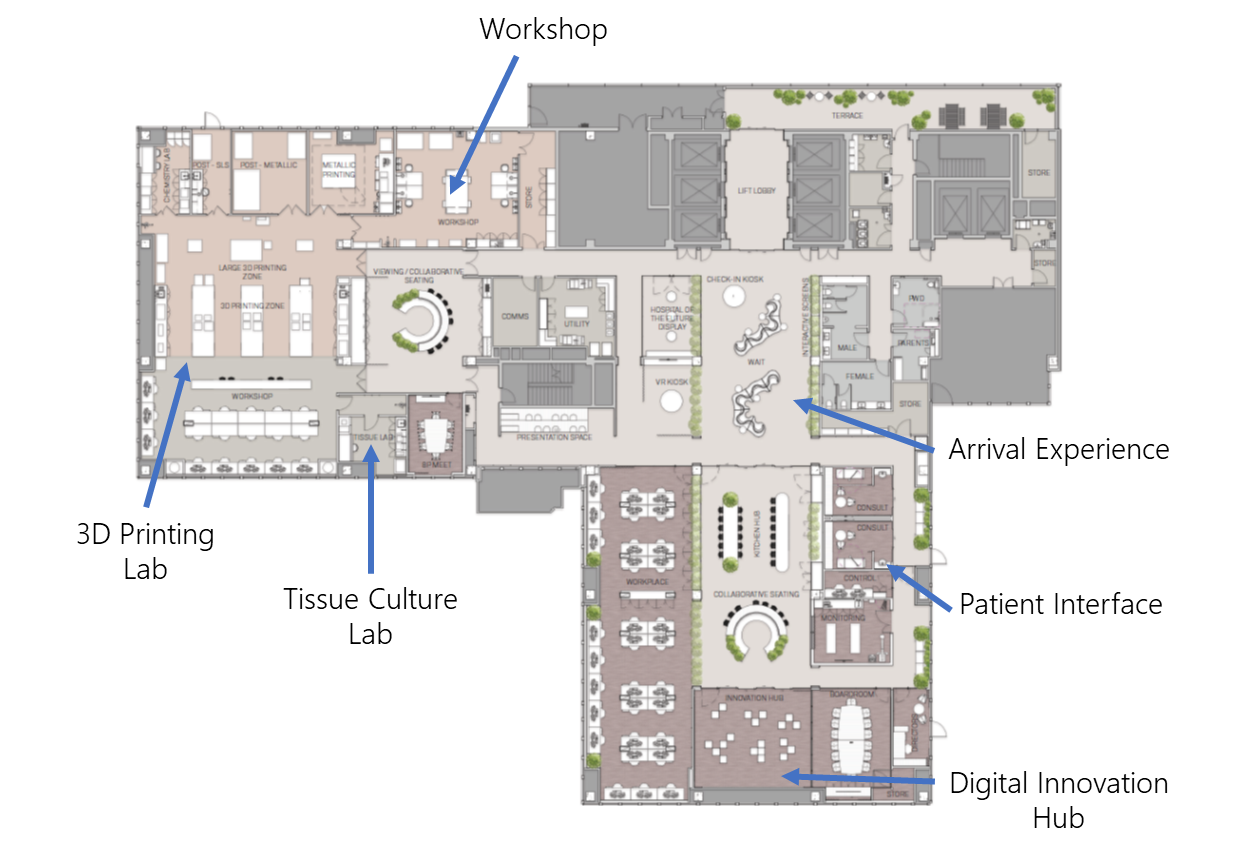
Located on 1,500 sqm within the Royal Brisbane and Women’s Hospital and the Herston Precinct, the Herston Biofabrication Institute brings together and promote open innovation and collaboration between clinicians, scientists, researchers, engineers, consumers and industry around grand challenges that will transform the surgical craft. We anticipate that within 10 years, biofabrication technologies will form a seamless part of the patient care pathway.



Equipment and facilities
- Stratasys J750 Digital Anatomy Printer multicolour and multimaterial 3D printer
- SLS 3D Systems sPro60: durable thermoplastics, flexible materials, Class VI biocompatible.
- Production-scale SLA resin 3D printers and Asiga DDP printers.
- Desktop printers: Markforged Mark 2, FormLabs Form 3, Ultimaker 2+ and S3, Prusa i3S multi-material.
- Tissue culture laboratory and Cellink BioX hydrogel 3D printer.
- Full mechanical workshop: CNC mill, laser cutter, lathe, band saw, drill press, sander, and more.
- Scanning and visualisation: Artec Leo surface scanner, Oculus Quest virtual reality suite.
- Philips Pulsera 3D mobile fluoroscopy system, Medtronic stealth station, ultrasound, surgical microscope.
- Software: Solidworks, Materialise Mimics, 3-Matic, Geomagic.
Medical Devices & Quality Assurance
Metro North Health is the sponsor and manufacturer of Class I medical devices in accordance with the Therapeutic Goods (Medical Devices) Regulations 2002.
In 2022, HBI manufactures the following devices for Queensland Health facilities:
- Surgery: patient-matched anatomical models for surgical planning.
- Cancer care: patient-matched radiation therapy bolus and brachytherapy models.
- Others: face shields and custom-made medical devices.
Contact our Biomedical Engineers: hbidevices@health.qld.gov.au
Metro North Health, Herston Biofabrication Institute (HBI)
Level 12, Block 7
Royal Brisbane and Women’s Hospital
HERSTON QLD 4029
Email: hbi@health.qld.gov.au
